Most drinking water plants use either surface water (lakes, rivers, and streams) or groundwater as their point source of water for treatment. Surface water is typically lower in mineral content, resulting in lower conductivity/TDS readings. Groundwater that has percolated through limestone, dolomite, or gypsum, and generally has a higher mineral content than surface water. However, there are sources of groundwater that are also very low in mineral content.
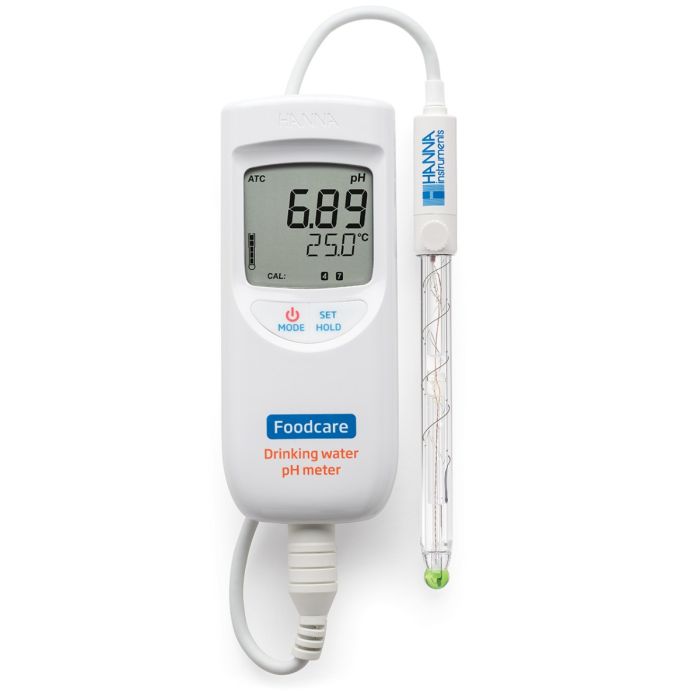
The HI99192 uses the FC2153 amplified pH electrode with glass body. This specialized electrode offers numerous features that improve pH testing in drinking water. An integrated temperature sensor allows for temperature compensated pH measurements without the need for a separate temperature probe. The probe’s spheric sensing tip has a wide surface area for measurement in aqueous solutions.
An integral part of any pH electrode is the reference junction. The reference junction is a part of the electrode that allows for the flow of ions located in the reference cell into the sample being measured. The ions provide for an electrical connection between the reference electrode and the indicating electrode. A standard pH electrode will use a single ceramic junction that allows for 15 to 20 μL/hour of electrolyte to flow.
The FC2153 has three ceramic junctions providing for 40 to 50 μL/hour of electrolyte to flow. This increased flow provides a greater continuity between the reference electrode and the indicating electrode making it suitable for water of low ionic strength. To optimize the flow from the electrode the refill cap should be unscrewed so that it is open. This allows for positive head pressure to be created allowing for the electrolyte to flow more easily into the sample.